Primary carcinoid tumor of the lung
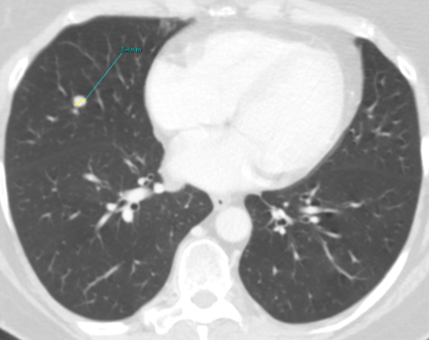
Fig. 1a
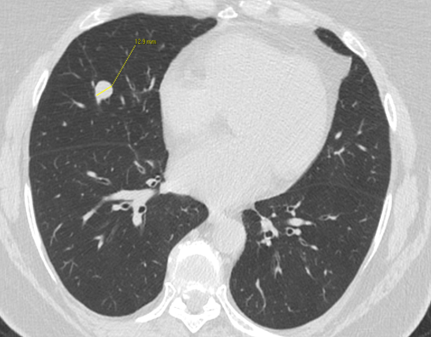
Fig. 1b
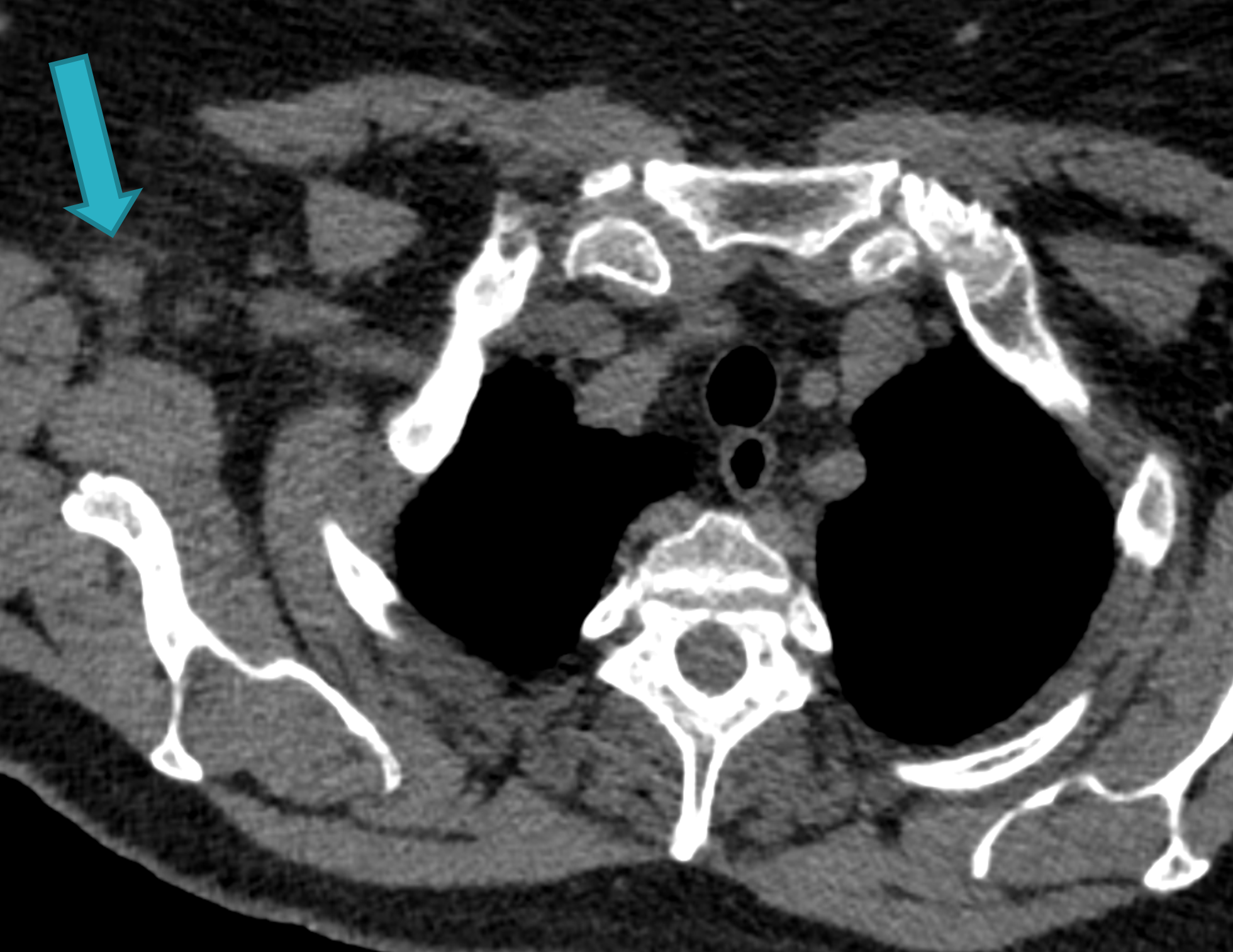
Fig. 1c
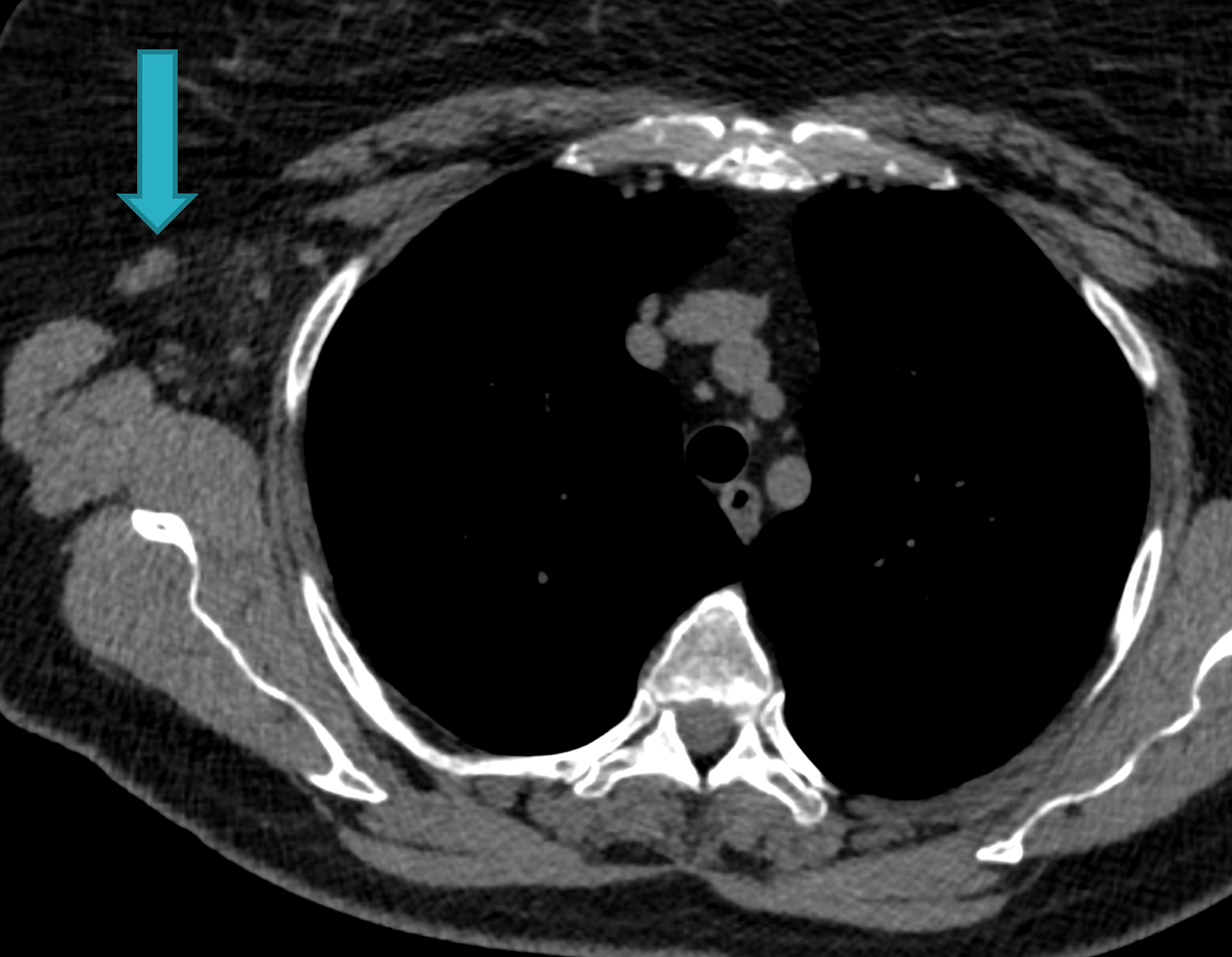
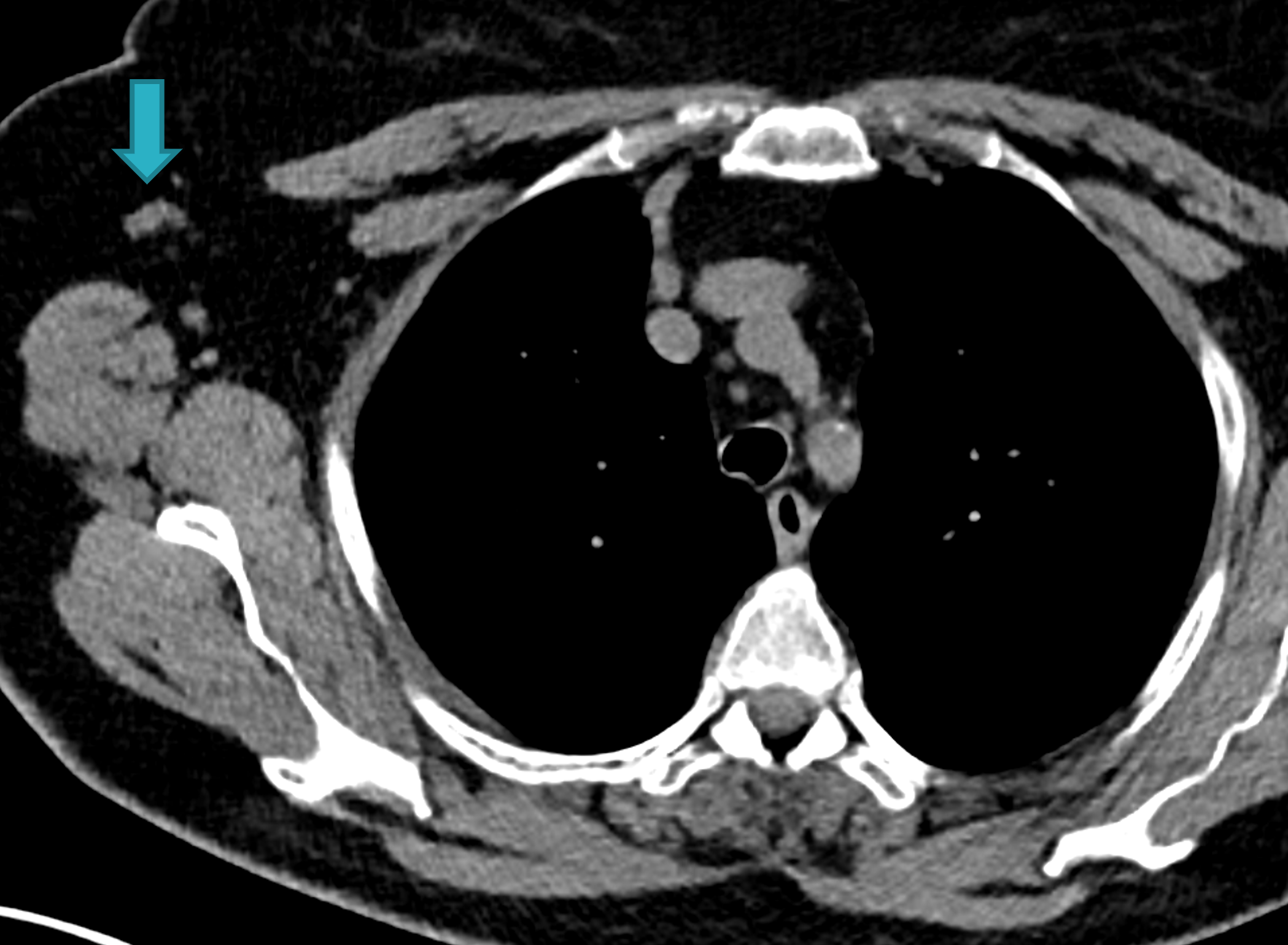
Fig. 1d
Fig. 1: Unenhanced CT examinations performed by a non-smoker 71-year-old woman during follow-up for a middle lobe pulmonary nodule.
The nodule was incidentally discovered during a CTU performed for urolithiasis. Over time, the nodule demonstrated stable smooth margins, hypodense appearance (mean HU 20) and no calcifications. Nodule’s size remained unchanged from 2011 to 2015, measuring about 8 mm (Fig.1a). Consequently, follow-up was concluded, considering the nodule might be pulmonary benign granuloma and no other investigations were performed.
The patient’s follow-up was performed following the 2005 Fleischner Society guidelines. Considering the patient as low-intermediate risk, she underwent several CT scans: the first three at about six to twelve months from each other and the last after about thirty months. The current Fleischner Society guidelines (2017) state the same indications as those published in 2005 for low risk patients for a single solid nodule measuring 6-8 mm.
In 2017 the patient was diagnosed with asthma. In 11/2019 the patient performed an unenhanced chest CT for acute asthma. The known pulmonary nodule was stable in morphology but increased in size, measuring 13 mm (b). In addition, some enlarged lymph nodes were observed in the right axilla (blue arrows, c and d).
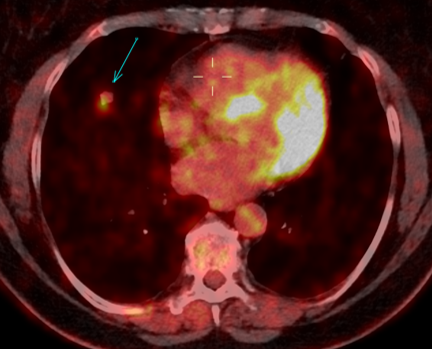
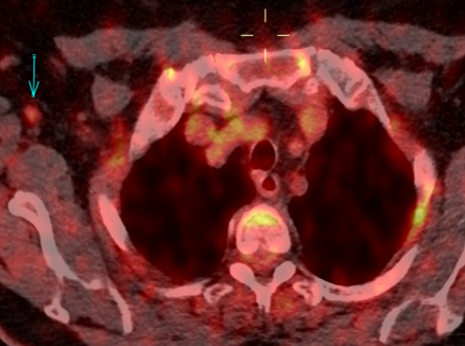
Fig. 2a
Fig. 2b
Fig. 2: 18F-FDG-PET/CT performed in 03/2020. Axial 18F-FDG-PET image show FDG uptake of the nodule (a), with an SUV max of 5.4. The axillary lymph nodes demonstrate a faint uptake, with an SUV max of 1.8 (b).
Following PET findings, the patient underwent a surgical resection of the pulmonary nodule (06/2020). The histological examination showed positivity for synaptophysin, cytokeratin CAM 5.2, AE1AE3 and chromogranin A, with a final diagnosis of typical pulmonary carcinoid.
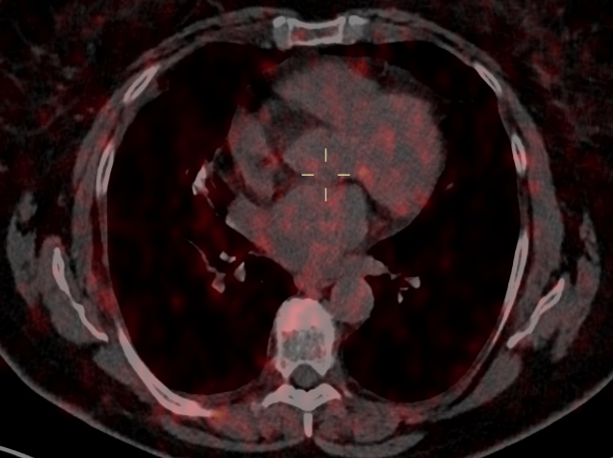
Fig. 3a
Fig. 3b
Fig. 3 – 68GA-DOTATOC-PET was performed for follow-up in 12/2020: no significant uptake in pulmonary parenchyma and in the right axillary nodes was noted.
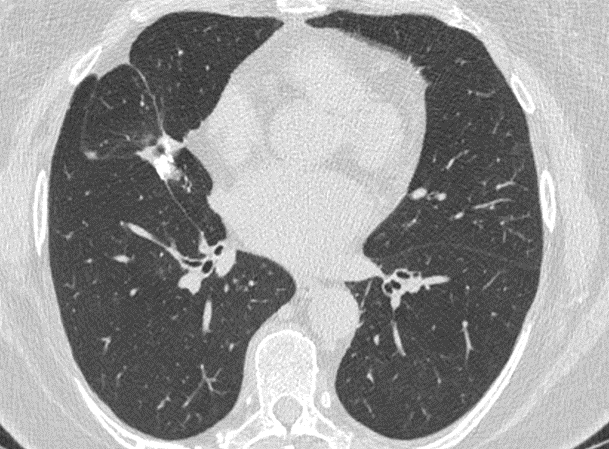
Fig. 4a
Fig. 4b
Fig. 4 – Unenhanced CT examination performed for follow-up in 02/2022. No evidence of disease recurrence with stable post-operative scar in the middle lobe. The right axillary lymph nodes did not show any sign of malignancy.
Summary:
- Primary carcinoid tumors of the lung are a rare neuroendocrine epithelial tumor (NET), with prevalence of 0,5-1% of primary lung cancers. They can be divided in two types, according to the degree of differentiation: typical and atypical carcinoids.
- Lung NETs are classified in: well differentiated (G1) or typical carcinoid, moderately differentiated (G2) or atypical carcinoid and less differentiated (G3) distinguished in a small cell and large cell variants (SCLC and LCNEC).
- The etiology is not clear. Air pollutant and some chemicals seem related to carcinoid. Tobacco smoking is correlated with atypical carcinoids but not with typical ones. Also having a family history of carcinoid tumors and MEN1 represent risk factors.
- Probably carcinoids develop from tumorlets, which are benign islets of hyperplastic neuroendocrine cells that grow in the context of areas of scars or inflammation. Most tumorlets remain stable for long time, but in some cases they can evolve into typical carcinoids, with low grade of malignancy.
- Pulmonary carcinoid can occur at any age from 5 to 90, but it occurs more frequently in the fifth decade and with a higher incidence in women.
- Most common symptoms are coughing or wheezing, hemoptysis and asthma. Small carcinoid tumors may be asymptomatic but some people presents symptoms related to carcinoid syndrome (high blood pressure, weight gain, facial flushing).
- Atypical carcinoid has a worse prognosis than typical carcinoid, with a 5-years survival ratesof 60% and 90% respectively.
- CT represents the gold standard for identifying carcinoid tumors, being in the majority of cases incidentally detected in studies performed for other clinical reasons. In most cases the nodule/mass on CT is well defined, with smooth margins, sometimes lobulations and central calcification.
- 68GA-DOTATOC-PET can differentiate carcinoid from other lung tumors, because of somatostatin receptors that are specifically present in carcinoid tumor cells.
- PET with 68GA-DOTA-peptides (DOTATOC, DOTANOC and DOTATATE) presents a higher sensitivity for well-differentiate lung NETs, such as typical carcinoid (G1). 18F-FDG-PET is more suitable for less differentiated lung NETs, such as atypical carcinoid (G2), LCNEC and SCLC (G3). However, the use of these radiopharmaceuticals should be considered complementary for a better evaluation of patients with pulmonary NETs.
- A new sperimental radiopharmaceutical, SiFAlin-TATE, has been recently tested, instead of 68GA-DOTA-peptides, for the diagnosis of well-differentiated NETs but results are still preliminary.
This Case was kindly provided by:
Dr. Usai Jessica (resident)
School of Medicine, University of Milano-Bicocca, Milan
Department of Radiology, Papa Giovanni XXIII Hospital, Bergamo – Italy
Dr. Bonaffini Pietro Andrea (staff)
School of Medicine, University of Milano-Bicocca, Milan
Department of Radiology, Papa Giovanni XXIII Hospital, Bergamo – Italy
Dr. Valle Clarissa (staff)
School of Medicine, University of Milano-Bicocca, Milan
Department of Radiology, Papa Giovanni XXIII Hospital, Bergamo – Italy
Prof. Sironi Sandro (chief)
School of Medicine, University of Milano-Bicocca, Milan
Department of Radiology, Papa Giovanni XXIII Hospital, Bergamo – Italy